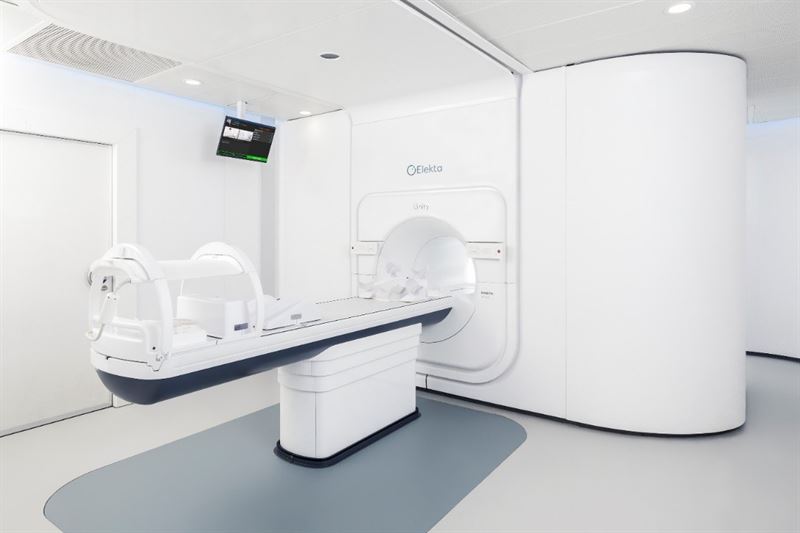
Elekta Unity MR-Linac receives clearance by China’s National Medical Products Administration
BEIJING, August 21, 2020 – Elekta (EKTA-B.ST) today announced that the world’s first high-field 1.5T MR radiation therapy system, Elekta Unity, has received clearance from the National Medical Products Administration (NMPA), clearing the technology for clinical use in China.
Elekta Unity is a state-of-the art MR-Linac that is setting a new standard for personalized adaptive radiation therapy. Elekta Unity provides the ability to reshape the dose based on daily changes in shape, size and position of the tumor and surrounding healthy anatomy and then enables accurate dose delivery with real-time visualization of the tumor. The system combines a Philips high-field 1.5T MRI scanner with a best-in-class 7MV linear accelerator and breakthrough online dose replanning software that are fully integrated to enable adaptive radiotherapy and real-time target monitoring.
Elekta Unity has followed strict clinical trials in top Chinese hospitals for nearly two years after its introduction, according to new NMPA guidelines. The system has the potential to significantly improve outcomes for people living with cancer and reduce side effects.
Cancer is a leading cause of death in China and the government’s Healthy China 2030 Planning Outline provides for comprehensive investments and solutions to improve current healthcare conditions. Anming Gong, Elekta’s Executive Vice President Region China, says: “The clearance to use Elekta Unity in China is in perfect harmony with these goals. Elekta Unity not only has the potential to transform accuracy, efficiency and clinical outcomes in radiation therapy but to also provide extraordinary potential for ‘hard to treat’ cancers, such as lung, liver, pancreas and colorectum cancers, which are among the leading types of cancer in China.”
According to GLOBOCON[1] statistics, an estimated 4.3 million new cancer cases and 2.9 million new cancer deaths occurred in China in 2018. With NMPA approval, many of these people affected by cancer will be offered a new hope for improved outcomes of their treatment.
To learn more, visit elekta.com/Unity.
Elekta Unity is CE marked and 510(k) cleared and has received regulatory clearances in China, Japan, Canada and Australia, among many other countries. Not commercially available in all markets.
# # #
For further information, please contact:
Mattias Thorsson, Head of Corporate Communications and Public Affairs
Tel: +46 70 865 8012, e-mail: Mattias.Thorsson@elekta.com
Time zone: CET: Central European Time
Cecilia Ketels, Head of Investor Relations
Tel: +46 76 611 76 25, e-mail: cecilia.ketels@elekta.com
Time zone: CET: Central European Time
This is information that Elekta AB is obliged to make public pursuant to the Securities Markets Act. The information was submitted for publication at 11:30 CET on August 21, 2020. (REGMAR)
About Elekta
For almost five decades, Elekta has been a leader in precision radiation medicine. Our more than 4,000 employees worldwide are committed to ensuring everyone in the world with cancer has access to – and benefits from – more precise, personalized radiotherapy treatments. Headquartered in Stockholm, Sweden, Elekta is listed on NASDAQ Stockholm Exchange. Visit elekta.com or follow @Elekta on Twitter.
[1] Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.